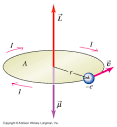
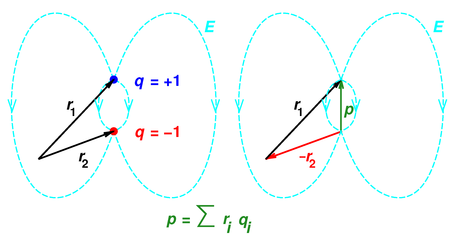
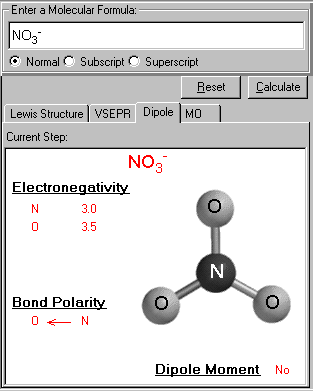
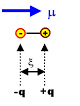
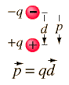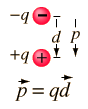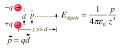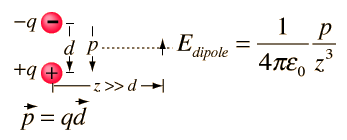Dipole moment : Videos
Dipole moment : Photo Gallery
Dipole moment : Latest News, Information, Answers and Websites
magnetic moment: Definition from Answers.com
magnetic moment n. The product of the pole strength of a magnet and the distance between the poles. ... The dipole component of an objects magnetic field is symmetric about the ...
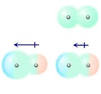
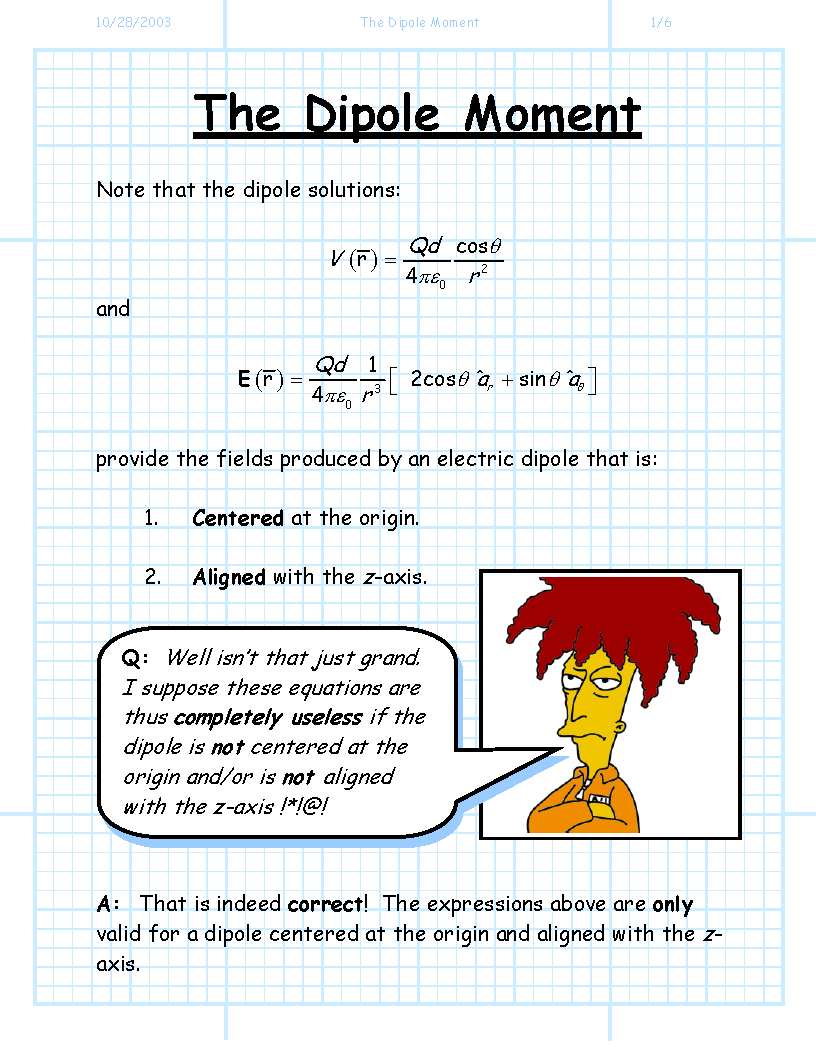
Dipole moment
basic introduction to explain the resolve of the dipole moment dipole moment and the polarization of the dipole moment method solution of a basic presentation to belong AB2 molecules, CO2 in the μ = 0, it can be refereed ...
Wikipedia:Dipole - Global Warming Art
For the current loop, the magnetic dipole moment points through the loop (according to ... The dipole moment of the bar magnet points from its magnetic south to ...
How do you tell which molecule has the largest dipole moment?
Are there specific characteristics? Bigger molecule, more electrons, ect?
For example, Which of the following molecules has the largest dipole moment?
a) H2O2
b) HCl
c) SO2
d) NO
e) N2
Thanks!
Answer: the dipole moment is a measure of the disproportionate distribution of electrons in the molecule. it is also affected by the shape of the molecule. net molecular dipoles can be zero even if the individual bonds are polar. CO2 is a good example of this, as it it is a linear molecule.
of the list you gave, the HCl will have the largest dipole moment because of the electron affinity of the chlorine over the hydrogen. I would place the peroxide next.
Category: Chemistry
Why does fumaric acid not have a dipole moment?
Maleic acid has a dipole moment, but the closely related fumaric acid, a substance involved in the citric acid cycle by which food molecules are metabolized, does not. Explain.
Answer: Fumaric acid has a point symmetry which maleic acid doesn't have (the C=C bond being in Cis configuration).
That symmetry causes that the distribution of charges is even, contrary to maleic acid, and the molecule is apolar.
Category: Chemistry
What would be the dipole moment on these?
I thought I had a clear understanding of dipole moment, however, I now see I dont. can anyone explain what the dipole moment is on the following and how to get that answer?? Thanks!
BCl3 BIF2 BClF2
Simon- thank you that was helpful. So in terms of the most or least dipole moment which of these would have the most dipole moment?
Answer: All the molecules you have listed are trigonal planar.
BCl3 doesn't have an overall dipole moment because all the individual B-Cl bond dipoles cancel out.
BIF2 has a dipole moment pointing between the 2 B-F bonds
BClF2 also has a dipole moment pointing between the 2 B-F bonds
To determine the dipole moment, look at electronegativity differences. Because F is the most electronegative halogen in each case here, this is where the dipole moment will point towards.
Difficult to explain without diagrams but hope that's OK!
Category: Chemistry
Magnetic dipole moment – Wikibooks tiếng Việt
The magnetic moment of a system is a measure of the magnitude and the direction of its magnetism. ... This is the basis on which the unit of magnetic dipole moment is defined. ...
What happens to a dipole moment if the molecule is non-polar?
i need to draw a 3-d sketch of carbon tetrachloride but it is a non-polar molecule and im not sure how to draw the dipole moment of each bond.
Answer: The molecule has dipole moments between carbon and each chlorine,
but since they cancel out, the net dipole moment is zero.
Category: Chemistry
cankler » Blog Archive » RMIT's Dr Madhu Bhaskaran Piezoelectric ...
The nature of the piezoelectric effect is closely related to the occurrence of electric dipole moments in solids. The latter may either be induced for ions on crystal lattice sites with asymmetric charge surroundings (as ...
What is the dipole moment of this pair of charges?
Two point charges, 1.4 pC and −1.4 pC,
are separated by 8 μm.
What is the dipole moment of this pair of
charges?
Answer in units of C · m.
Answer: p = qd = 1.4x10^-12(8x10^-6) = 11.2x10^-18 C m
Category: Physics
Dipole moment - Wikipedia, the free encyclopedia
Bond dipole moment, the measure of polarity of a chemical bond ... Magnetic dipole moment, the measure of the magnetic polarity of a system of charges ...
Why is the Electric Dipole moment vector pointing in the direction -q to +q?
I understand that the lines of force move outward from a positive charge. It will be directed inwards if it is a negative charge.
That said, I expected the electric dipole moment vector in the direction +q to -q. But I could see the opposite in all the text books.
Please advise why this is written so?
Answer: let me try to explain it this way. Suppose i have an electric dipole that is placed in an external electric field E(vector) :
..... + .----------->E
.../....----------->E
../.....----------->E
_......------------>E (like this figure!!)
Now, scientists try to calculate the energy stored in the dipole due to its position and configuration in the external field.
When you learn about angular motion, you probably know this equation that describe the potential rotational energy : delta U = U(final) - U(initial) = (integral from θ(initial) to θ(final) of the torque)
= ∫τ dθ
Let's just skip the calculation to determine the energy. But what we really need now is how to find the torque in order to estimate U.
From the figure above, you might see that we assume the field points from the left to the right, there fore it will exert a force on the two charges. For the positive charge, the force is to the right and for the negative charge, the force is to the left, but the charges attract each other.Thus they cause the electric dipole to rotate around an axis that is perpendicular to the page and pass through the center of the line connects the charges.
The torque vector is the cross product of the displacement vector (r) and the force vector (F)
then τ = r X F = F*r*sin(θ) = F*(a)*sin(θ) (since we let a=r is half the distance from the negative charge to the positive charge and it's exactly the distance from the center to each point charge)
And the total torque is : (total)τ = τ 1(positive) + τ 2(negative) = 2F*a*sin(θ)
But the electric force F = qE, we substitute it into the equation to get:
(total)τ = 2qEasin(θ), rearrange it, we have : (total)τ = (2qa)E(sin(θ))
Since it's easy to calculate E and θ, we let E and sin(θ) out and group two quantity a,q and the coefficient 2 as one quantity called the electric dipole moment p
So now, p = 2aq
That's how we got the magnitude of the electric dipole moment. But there's a problem since we realize that the torque (total)τ and E are all vector quantities. So that means the electric dipole moment p must also be a vector quantity so that we can perform the cross product calculation.
(total)τ(vector)= p(vector) X E(vector)
Here we have two choices, one is p points from the negative to the positive and the other is the opposite.
Let me remind you that since the electric field points from left to right, the e.dipole will definitely rotate clockwise
The toque is the vector that lies along the axis of rotation and since we have an international convention that if it rotates clockwise, the angular velocity is negative and if it rotates counter-clockwise then the angular velocity is positive
if I use the right hand rule to find the direction and sign of the angular velocity , i find that the angular velocity vector points into the page and because τ= I*(angular velocity), the torque must points in the same direction, which is into the page.
It's very clear now that τ(vector) = p(vector) x E(vector). We find the magnitude by performing this calculation: τ = pEsin(theta) and determine the direction using the right hand rule .
As we discuss earlier, τ(vector) points into the page and E is to the right, thus the only solution for the direction of the electric dipole moment vector is from the negative charge to the positive charge(not the opposite one)!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
This works well with different configurations, too.
And with that, we have a complete definition of the electric dipole moment vector that would fit and be consistent with so many previous physics laws and equations.
Good luck with this!!!
Category: Physics
Why must a bond in a molecule undergo a dipole moment change when IR radiation is absorbed to be detected?
When performing infrared spectroscopy on a molecule, why must a bond in the molecule be undergoing a change in the dipole moment when the infrared radiation is absorbed? The absorbed is not detected if this does not happen. Why?
Answer: that is how the math work - the frequency of the vibration = freq of change of a dipole moment has to equal the freq of the light and the molecule can "couple" to the light. so symmetreic stretches are not absorbed.
Category: Chemistry
Dipole - Wikipedia Mirror
Dipoles can be characterized by their dipole moment, a vector quantity. ... For the current loop, the magnetic dipole moment would point through the loop (according ...
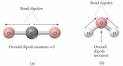
Ionic and Covalent Binding - Dipole Moments and Polar Bonds
The dipole moment is defined as the product of the total amount of positive or negative charge and the distance between their centroids The centroids of the ...
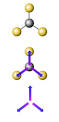
Dipole Moments
Dipole Moments. The strength of the dipole depends upon the. difference in the ... Dipole Moments. A 4th example, CH. 4. The hybridization about C is sp. 3 ...
Dipole Moment | Newz of Today
More accurate to discover an electric dipole moment of an electron experiment has discovered no [1]. Do not miss also the article of the news and views of this work in the same issue of nature. ...
What is the direction of the dipole moment of these bonds?
1. Using an arrow in which the arrowhead is negative, show the direction of the dipole moment of the bonds indicated.
H3C---------Br
I----------Br
(H3C)2N-------------H
Thanks! Will rate for a correct answer!
Answer: in the first one it will be toward bromine bc its more electronegetive
in 2nd it will be towards br
in third it will be towards nitrogen
Category: Chemistry
Molecular Dipole Moments
Such molecules are said to be polar because they possess a permanent dipole moment. A good example is the dipole moment of the water molecule. ...
The Reference Frame: Electron dipole moment below 1e-27 e.cm ...
dipole moment. There are at least two types of torque generated between the permanent dipole magnetic moment of an electron spin in a molecule or ion and a circularly polarized electromagnetic field. ...
To have a dipole moment an element has to have a lone pr of electrons?
I want to know specific detail on a dipole moment.
Answer: It's molecules that have dipoles. You need to review your bonding basics.
Category: Chemistry
YouTube - Dipole Moment
Oct 5, 2009 ... Drawing Lewis structures and using VSEPR to determine if a molecule is polar or nonpolar (i.e., does the molecule have a dipole?)
Dipole moment - Wikipedia, the free encyclopedia
Dipole moment may refer to: Electric dipole moment, the measure of the electrical polarity of a system of charges; Transition dipole moment, the electrical ...
How does a molecules dipole moment separate its multiple forms?
A question Im working on asks of C2H2Br2, "How can the dipole moment be used to separate the two forms of C2H2Br2?"
And Im really confused as to what the question is even asking. Any help in understanding or answering the question is appreciated. Thanks!
Answer: Be sure you understand what a dipole moment is first of all. Have you ever seen a model of an water molecule? It's sort of a wide V shape right? Now, you need to develop a sort of intuition about the ways a molecule will behave based around it's affinity (love of) electrons. Oxygen molecules have a much greater affinity for electrons than hydrogen, so, those electrons, to put it simply are going to move towards the oxygen and away from the hydrogens. So, the one oxygen molecule will pull those electrons closer giving it a partial negative charge (a dipole moment) and partial positive charges to the hydrogens (the other dipole moments). So it sounds like your question has something to do with this principle, remember, partial positives will repel partial positives, and partial opposites will attract. Look at it's structure if you can. If you think about water, you'll realize this is why it takes so much energy to boil, the molecules are holding on to each other! Think about the chemical principles behind your question.
Category: Chemistry
Dipole - Wikipedia, the free encyclopedia
Dipoles can be characterized by their dipole moment, a vector quantity. ... For the current loop, the magnetic dipole moment would point through the loop (according ...
Physics and Physicists: No Electric Dipole Moment For Electron Yet
The most accurate experiment to detect any electric dipole moment for an electron has detected none[1]. Don't miss also the News and Views article of this work in the same issue of Nature. This experiment has received ...
Net dipole moment
A fellow brother in Arizona has enjoyed a few weeks about 110 F and daytime temperatures are 60 F at night. Relative humidity is difficult to 0%. The air is...
Dipole - Wikipedia, the free encyclopedia
Dipoles can be characterized by their dipole moment, a vector quantity. For ...
Molecular Dipole Moments
Such molecules are said to be polar because they possess a permanent dipole moment. A good example is the dipole moment of the water molecule. ...
Dipole - Wikipedia, the free encyclopedia
For the current loop, the magnetic dipole moment points through the loop (according to ... The dipole moment of the bar magnet points from its magnetic south to ...
New technique narrows electron dipole moment - physicsworld.com
But if the electron does have a non-zero electric dipole moment (EDM), it would have profound implications and point to new physics. Now, Jony Hudson and colleagues at Imperial College London have made the most precise ...
Dipole definition of Dipole in the Free Online Encyclopedia.
Information about Dipole in the Columbia Encyclopedia, Computer Desktop Encyclopedia, computing dictionary. dipole antenna, dipole moment, dipole magnet ...
The Reference Frame: Electron dipole moment below 1e-27 e.cm at 90 ...
Note that as early as in 2005, this blog was talking about planned experiments that wanted to measure the electric dipole moments at the accuracy 100 times more accurate than the figure above. ...
Dipole - wikidoc
Bar magnet dipole moment. In physics, there are two kinds of dipoles ... Dipoles can be characterized by their dipole moment, a vector quantity. ...
Answers.com - What is a dipole moment
Chemical Bonding question: What is a dipole moment? AnswerDipole moment is the measure polarity of a polar covalent bond .In language of physics it can be ...
Electric dipole - encyclopedia article - Citizendium
In this definition a dipole is a vector depending on the characteristics of the ... Often, especially in chemistry, this size (a scalar) is also referred to as "dipole moment" ...
Dipole Moment
Burdick & Jackson solvents are arranged in order of increasing dipole moment, the mathematical product of the distance between the centers of charge in the ...
New technique narrows electron dipole moment - physicsworld.com
May 26, 2011 ... But if the electron does have a non-zero electric dipole moment (EDM), it would have profound implications and point to new physics. ...
Dipole moment????????????
Could anyone help me figure out how to draw the dipole moment of Ortho-dichlorobenzene and also Para-dichlorobenzene. Im guessing the dipole moment of Para molecule is zero since its symmetrical. Thanks
Answer: You are correct, zero, Para has the halogen at either end of the molecule, so is symmetrical. Remember, Ortho, Meta, Para correspond to 2, 3, 4 position on benzene. Dipole moments come into play in Raman and IR, hopefully you aren't doing that as its not easy.
Category: Chemistry
Dipole - New World Encyclopedia
A dipole can be characterized by its dipole moment, a vector quantity. ... For a magnetic dipole, the magnetic dipole moment is the strength of either magnetic pole ...
What is the magnetic dipole moment in MRIs?
Im learning about how MRIs work, and Im having trouble understanding what exactly the magnetic dipole moment is. Can anyone answer this in a simple way?
Answer: a measure of the magnetic strength of a magnet or current-carrying coil, expressed as the torque per unit magnetic-flux density produced when the magnet or coil is set with its axis perpendicular to the magnetic field. Symbol m j Also called magnetic moment
Category: Physics
What factors determine whether or not a molecule has a dipole moment?
What does it mean to say a molecule is polar? What factors determine whether or not a molecule has a dipole moment?
I understand what a polar molecule is but I dont know what the dipole movement factors are.
Please help.
Thank you.
Answer: Any molecule that, as a whole, has an asymmetrical charge distribution, will be polar (have a dipole moment)
Any bond between two elements with different electronegativities will have some ionic character and thus produce a dipole moment. The spatial direction and strengths of these dipole moments will average out to form the molecule's dipole moment.
A few examples:
Water has a dipole moment because it is a bent molecule.
CO2 does not have a dipole moment, because it is linear and the dipole moments of the two C=O bonds cancel.
BCl3 is flat and triangular, so the dipole moments of the 3 B-Cl bonds cancel.
NH3 and NCl3 have a trigonal pyramid shape, so the dipole moments of their 3 bonds do not cancel.
Category: Chemistry
Groove|Asia Directory: Dipole
For the current loop, the magnetic dipole moment points through the loop (according to ... The dipole moment of the bar magnet points from its magnetic south to ...
Dipole
Dipol Dipole In physics, there are two kinds of dipoles (Hellènic: di(s)- = twi- and pòla = pivot, hinge). An electric dipole is a separation of
dipole moment: Definition from Answers.com
dipole moment n. The product of either charge in an electric dipole with the distance separating them
why does the experimental value of dipole moment different to the calculated one ?
when the dipole moment of a molecule is calculated using the formula, it is always different to the experimental value so what is the scientific explanation behind this ?
Answer: Bond dipole moment
From Wikipedia, the free encyclopedia
Jump to: navigation, search
The bond dipole moment is a measure for the polarity of a chemical bond within a molecule. The bond dipole μ is given by:
\mu = \delta \, d.
The bond dipole is modeled as, +δ — δ- with a distance d between the partial charges +δ and δ-. It is a vector, pointing from minus to plus,[1] that is parallel to the bond.
Chemists generally measure electrical dipole moments in debyes, represented by the symbol D. The SI unit for dipole moment is the coulomb-meter (1 C m = 2.9979 1029 D), δ is the amount of charge in coulombs, and d is in meters.
For a complete molecule the total molecular dipole moment may be approximated as the vector sum of individual bond dipole moments. Often bond dipoles are obtained by the reverse process: a known total dipole of a molecule can be decomposed into bond dipoles. The reason for doing this is the transfer of bond dipole moments to molecules that have the same bonds, but for which the total dipole moment is not yet known. The vector sum of the transferred bond dipoles gives an estimate for the total (unknown) dipole of the molecule.
The Bond Dipole is two atoms in a bond, such that the electronegativity of one atom changes and draws the electrons towards the other, causing a partial negative charge. There is an increase difference in polarity, and an increase in dipole.
Category: Chemistry
Is there a quick way to determine the dipole moment?
Short of drawing out the Lewis structures or simply memorizing a vast amount of molecular structures, is there a way to determine if a molecule has a non-zero dipole moment just from its formula?
Answer: "Short of drawing out the Lewis structures", no.
E.g. SO2 does, CO2 doesn't
Category: Chemistry
Of the following molecules, which would have a measurable dipole moment for the molecule?
HCCH
SF6
FOOF
F2CCF2
PF5
Also, What is the percent ionic character for an compound with the general formula A+B- compound whose internuclear distance is 1.36 and whose observed dipole moment is 1.2 Debye?
Answer whichever one you can please!!! THANKS!
Answer: PF5 has a measurable dipole moment for the molecule.
The others are symmetric.
Category: Chemistry