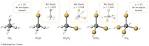
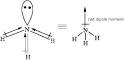
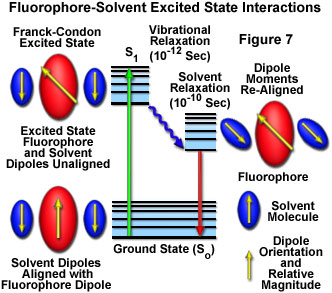
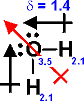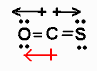Net dipole moment : Videos
Net dipole moment : Photo Gallery
Net dipole moment : Latest News, Information, Answers and Websites
Selecting Adhesives | Acid-Rain and Benefits of Magnesium Oil
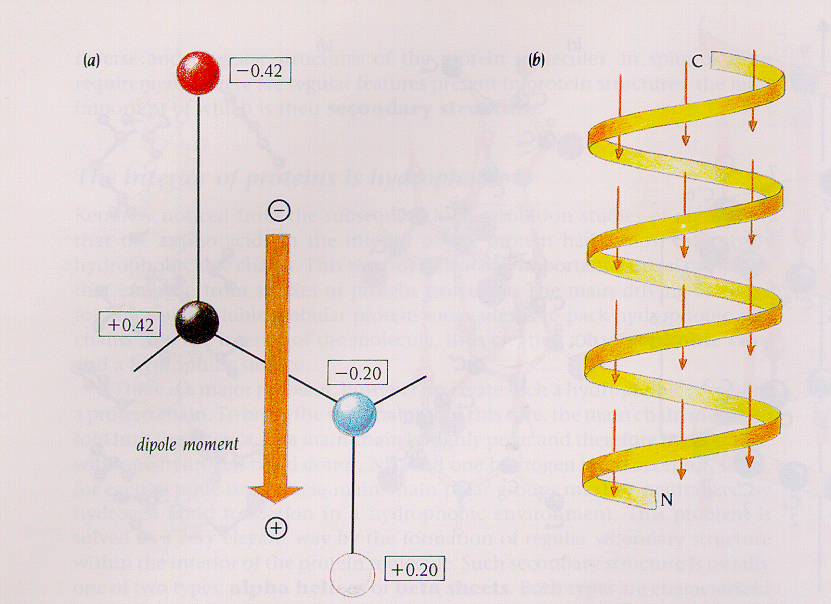
Secondary bonding includes dipole–dipole bonding (the interaction of molecules that have a permanent net dipole moment) and hydrogen bonding (an interaction that occurs when a hydrogen atom is bonded to an N, O, ...
Dipole - wikidoc
Dipoles can be characterized by their dipole moment, a vector quantity. ... out a net dipole moment, the value is set at 0. The highest dipole moments are in the ...
What are some common difficult dipole-moment questions in organic ...
well,they can ask you about the lipophilicity of drugs and why some drugs have short period of action.this is because lipophilicity of drugs depends on the dipole moment of the drug.if the drug has a high dipole moment,then it is more ...
Attraction-driven aggregation of dipolar particles in an external ...
are placed on the top of the net with random position and random direction of the dipole moments. The particles start to move at the same time when the net is immersed into the water letting the particles float. ...
If a molecule doesnt have a net dipole moment, but it has polar bonds, is it soluble in water?
If the bonds are polar but the vectors cancel out so there is no molecular dipole moment, would it be soluble in water? Why or why not?
Answer: no. Only polar molecules are soluble in water. This is because Water is a polar molecule [O has more electronegativity than H] so, the end of a polar molecule attracts the oppositely charged end of a polar water compound, and the molecule breaks down.
Category: Chemistry
Which of the following has a net dipole moment and why?
SCl4, PH3, BrCl3, and H2Se
I would really appreciate some help. Thanks!!!!
Answer: All of them have net dipole moments because all of them are polar.
Their overall polarity is due to their shape as created by the repulsion of the electron pairs (both bonding and non-bonding electrons).
The shapes are
SCl4 -- seesaw (irregular tetrahedron), AX4E
PH3 -- trigonal pyramid, AX3E
BrCl3 -- T-shaped, AX3E2
H2Se -- bent, AX2E2
---------------------------------
A = central atom
X = atom bonded to central atom
E = lone pair (non-bonding electron pair)
Category: Chemistry
net dipole moment?
Which of the following has no net dipole moment?
a. CH2O
b. CH2Cl2
c. CS2
d. PH3
e. H2S
Answer: You should draw a Lewis Dot Structure. A dead giveaway that a molecule has no dipole moment is symmetry -- i.e. no unmatched lone pairs, no positive charges on one end and negative charges on the other.
The only symmetrical molecule in your list is CS2. The central atom is carbon, and it's bonded to each sulfur by a double bond, so there's no lone electron pairs. It's a linear shape because of the double bonds, the two sulfurs cancel out any charges leaving a net dipole moment of zero.
Category: Chemistry
Dipole Moments
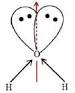
there is a net dipole since the dipoles do not. completely cancel. Dipole Moments. O. S. O. S ... balance yielding no net dipole in the vertical direction. ...
How do you find out if a molecule has a net dipole moment?
which of these molecules have net dipole moments?
XeCl2, ICl3, TeF4, PCl5, ICl5, XeCl4, SeCl6
and, if you can, could you explain what a net dipole moment is? THANKS!!!
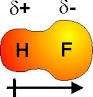
Answer: A dipole moment occurs when a molecule has a permanent charge separation δ+OOOOδ- it is said to be polar. First we determine the structure using VSEPR. We then place vectors (arrows) along the bonds in the direction of the most electronegative element in the bond (despite what the physicists would have us do) including lone pairs. If the vectors cancel there will be no net dipole even though the individual bonds are polar. If the vectors do not cancel the molecule will have a net dipole and will be polar. How this works will become apparent with the examples:
XeCl2: Xe had 8ve-, uses 2e- for Xe:F bonds. This leaves 6 e- or three lone pairs. It is an AX2E3 system linear: the ←F-Xe-F→ vectors cancel as do the Xe→: vectors. Molecule has no dipole moment.
ICl3 I has 7 ve-, 3 e- used for I:Cl bonds leaves 2lp: An AX3E2: T shaped. I-Cl vectors do not cancel (nor do the I→: vectors) so molecule has a dipole moment.
TeF4: AX4E, see saw: has a dipole moment
PCl5 AX5 trigonal bipyramid no dipole moment
ICl5 AX5E square pyramidal: has a dipole moment
XeCl4 AX4E2 square pyramidal (lp trans so vectors cancel, as do the trans Cl-Xe-Cl vectors): no dipole moment
SeCl6 AX6: octahedral, no dipole moment
Category: Chemistry
Chem II help! 10 Points! Having to do with Net Dipole Moment?
Which of the following molecules below has a net dipole moment?
a) BCl3
b) SiCl4
c) PCl3
d) Cl2
e) none
the answer is C.. but how?
Answer: Because Phosphorus Trichloride has a lone pair of electrons that directs the net dipole moment!
Category: Chemistry
Dipole - Wikipedia, the free encyclopedia
Dipoles can be characterized by their dipole moment, a vector quantity. ... out a net dipole moment, the value is set at 0. The highest dipole moments are in the ...
Ch. 5
The ClF bond is a polar covalent bond and the molecule has a net dipole moment (unlike CCl4 for instance where the bond dipoles cancel giving the ...
Net Dipole moment? Im confused! Please help!!!?
Ive tried this problem and read through my texts but I still dont know who to do this problem. If anyone can help with this it would be greatly appreciated!
Which of the following molecules have a net dipole moment? (There can be more than one)
CH3BR
CBr4
CH4
CH2Br2
CHBr3
Thanks so much for your help!
Answer: Dipole moment is a vector quantity. Its value in non zero between two different atoms.e.g C-Br. In a polyatomic molecule the net dipole moment is the vector addition of the individual bond dipole moments.The listed molecules are tetrahedral. The following molecules have net dipole moments(Non zero)
CH3Br, CH2Br2,CHBr3
The following have zero net dipole moments.
CBr4, CH4
You should know the geometry of the molecule. At the centre of a regular tetrahedron is C atom and the vertices are other atoms like Br,or H. A little imagination will tell you why CH4 has zero net dipole moment and CH3Br has net dipole moment.
Category: Chemistry
Dipole Moment | Law of attraction
@dasistdas Typically, one uses bond dipoles to find the net dipole moment qualitatively though it is also found experimentally. Qualitatively, one can compare the EN difference for each bond and draw a resulting dipole. ...
Magnetic dipole moment – Wikibooks tiếng Việt
Magnetic moment usually refers to its magnetic dipole moment, and quantifies the ... Any system possessing a net magnetic dipole moment will produce a dipolar ...
In the compound 1,4 - dihydroxy benzene or hydroquinone (common name) the net dipole moment is not = zero why?
The 2 OH molecules in hydroquinone are attached at the opposite ends of the benzene ring and their dipole moments should cancel each other out, resulting in the net dipole moment = 0. But this is not observed. There is some value of dipole moment present in hydroquinone. Why ??
Answer: These molecules are associated through hydrogen bonding with each other and the dipole moments of para hydroxy groups never cancel each other. They form a linear chain of molecules.
Category: Chemistry
Net Dipole moment? Please help!!!?
Ive tried this problem and read through my texts but I still dont know who to do this problem. If anyone can help with this it would be greatly appreciated!
Which of the following molecules have a net dipole moment? (There can be more than one)
CH3BR
CBr4
CH4
CH2Br2
CHBr3
Thanks so much for your help!
Category: Chemistry
Any of these have a net dipole moment?
I had to pick out the molecules with no net dipole moment, are all of these correct?
CF4
B(CH3)3
CF2Cl2
Here are diagrams of the molecules:
http://img156.imageshack.us/my.php?image=89368819bk4.jpg
Thanks, I went back to the electronegativity, although my professor said to regard halogens as practically the same... O_o
Answer: CF4 and B(CH3)3 both have no net dipole but CF2Cl2 would have a dipole. If you think about why CF4 has no net dipole (vector analysis adds to zero) you can see this. The fluorine atoms as the most electronegative element will have more pull on the electrons than the chlorine atoms resulting in a dipole pointing towards the side of the molecule where the fluorines are attached.
Category: Chemistry
Dipole - New World Encyclopedia
A dipole can be characterized by its dipole moment, a vector quantity. ... out a net dipole moment, the value is set at 0. The highest dipole moments are in the ...
The Electric Dipole Moment Vector -- Direction, Magnitude ...
The dipole moment is also the first moment of the charge distribution, ... can be called the overall charge, or the net charge, or the total charge, or simply "the" ...
Dipole moment - Wikipedia, the free encyclopedia
Bond dipole moment, the measure of polarity of a chemical bond ... Magnetic dipole moment, the measure of the magnetic polarity of a system of charges ...
Which molecule possess a net dipole moment?
Which molecule possesses a net dipole moment?
CH4
NH3
O2
Ar
none of the preceding
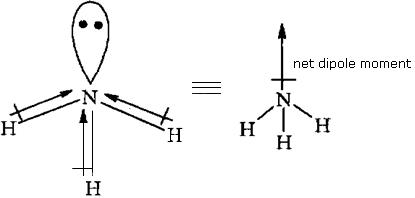
Answer: NH3 Due to the position of the lone pairs.
Category: Chemistry
Dipole - Wikipedia, the free encyclopedia
When the symmetry of a molecule cancels out a net dipole moment, the value is set at 0. The highest dipole moments are in the range of 10 to 11. ...
Dipole Articles & Dipole Websites at HealthHaven.com
The dipole moment of the bar magnet points from its magnetic south to ... out a net dipole moment, the value is set at 0. The highest dipole moments are in the ...
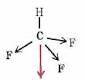
Is CH3Cl a polar molecule (a net dipole moment)?
I drew the shape of the molecule which is tetrahedral. 3 arrows are pointing outwards and 1 is pointing towards the central carbon atom. So, how do I know if there is a net dipole moment?
Thanks
What about CHCl3? Thanks!
Answer: Since carbon is more electronegative than hydrogen, the individual dipole moments will point from H to C. Cl is more electronegative than C, so that individual dipole moment points to Cl. This means there is a net dipole moment (the sum) in the same direction as the Cl atom, along the C--Cl axis.
Category: Chemistry
Chemistry 6 (9 am section) Spring 2000 Dipole Moments
Thus, the magnitude of our reference dipole moment is 4.8 D. We shall make ... dipole moment is correlctly calculated. using the pair of net charges ...
IB Biology/Chemistry: IB Chemistry, Stereoisomers, Geometric ...
Cis b/p high - polar (net dipole moment ) Cis m/p low - unable to pack (bend/kink) Trans b/p low - non polar (bond polarity cancel) Trans m/p high - pack together ( linear ) Packing causes molecules to align/pack closer ...
What is the difference between a dipole moment and a net dipole moment?
Answer: dipole moment is associatedd with a bond
net dipole moment is adding them vectorically
example CO2 O=C=O has strong dipole moments but no net dipole moment
Category: Chemistry
Net dipole moment hellppppppp??!!!! please!!!!!!?
Which of the following has a net dipole moment and why?
SCl4, PH3, BrCl3, and H2Se
Answer: Hey, you're in my class (: (Scerri, yah?)
They all have a net dipole moment because all of them are polar due to the presence and repulsion of the lone pairs that keep the angles from being the same which it keeps the dipole vectors from canceling out. Hoped that helped, I was having trouble on this too!
Category: Chemistry
Collaboration unveils unexpected ice formation - The Scarlet
In bulk ice or water, like that in a lake, stream or ice cube, the dipoles are all randomly oriented and the water has no net dipole moment. That's not the case with the new one-dimensional ice, though. ...
Answers.com - What is a dipole moment
Chemical Bonding question: What is a dipole moment? AnswerDipole moment is the measure polarity of a polar covalent bond .In language of physics it can be ...
Ionic and Covalent Binding - Dipole Moments and Polar Bonds
Dipole Moments and Polar Bonds. Any chemical bond results from the ... The dipole moment is defined as the product of the total amount of positive or ...
Does this molecule exhibit net dipole moment?
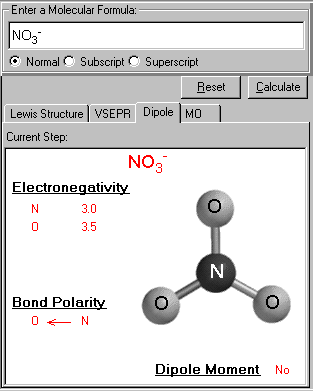
Using the valence Bond theory, and valence shell electron repulsion theory give a complete analysis of the type(s) of bonding, geometry, and bond angles in PO4 ^-3. Does this molecule exhbiti a net dipole moment?
Answer: use perhaps the Riemann's dzeta function
Category: Chemistry
Molecular Dipole Moments
To observe this intermediate behavior, we can examine molecular dipole moments. ... We might initially expect that molecules do not in general have dipole moments. ...
DoITPoMS TLP - Ferroelectric Materials - The dipole moment
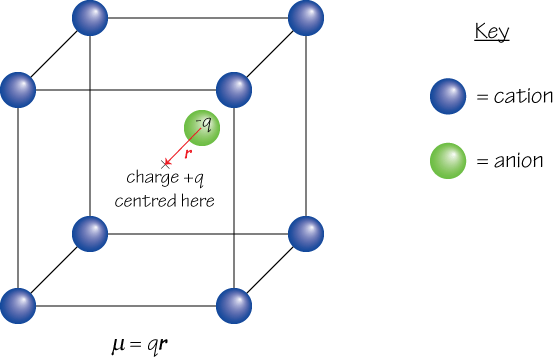
In a ferroelectric material, there is a net permanent dipole moment, which comes from the vector sum of dipole moments in each unit cell, Σμ. This means ...
lone pairs and dipole moments [Archive] - Physics Forums
Do lone pairs in a molecule have dipole moments? and does net dipole always go in the direction of the lone pairs? ...
net dipole moment, why is this the answer??!!?
Which of the following molecules has a net dipole moment?
a. BeCl2
b. SF2
c. KrF2
d. CO2
e. CCl4
correct answer: b. SF2
can someone please explain why b is the answer?
Answer: All the others are linear or symmetrical. SF2 is bent.
Category: Chemistry
Net dipole moment vector of CCl4?
would this be okay to say this regarding the net dipole moment vector of CCl4?
- CCl4 is nonpolar because its shape is perfectly symmetrical (tetrahedral shape) and the polar bonds cancel. Therefore the net dipole moment vector is 0.
or should i change my last sentence and say therefore the net dipole moment vector cancels
Answer: Either one would be fine. If I chose the second one I would end with "cancels to zero".
Category: Chemistry
Bond Polarity and Dipole Moment
Electronegativities, Bond Polarities and Dipole Moments -The electrons on the most ... all bond polarities are cancelled out. The net dipole moment is zero.
Net Dipole Moment
Net Dipole Moment. Water is the most common liquid on Earth, but scientists more study, the more surprises seem to find. This is nothing more true than with the University of Nebraska-Lincoln of Xiao Cheng Zeng and his ...
Magnetic moment - wikidoc
Specifically, magnetic dipole moment quantifies the contribution of ... Any system possessing a net magnetic dipole moment will produce a dipolar magnetic field ...
Animated Demonstration of Liquid Crystals Properties: Flexoelectricity
In the undistorted state, the dipole moments of the molecules are oriented with equal probability in opposite directions. They cancel each other and the net dipole density is zero. Let a nematic be made of pear or wedge ...
MRSEC | A Permanent Dipole Moment in Nanocrystals:
The Chicago Materials Research Center addresses fundamental scientific problems of ... atom has any net charge and thus no dipole moment exists (symmetric charge distribution) ...
dipole moment: Definition from Answers.com
dipole moment n. The product of either charge in an electric dipole with the distance separating them
Bond Polarity and Dipole Moment
The separation of positive and negative charges causes an electric dipole moment. ... all bond polarities are cancelled out. The net dipole moment is zero. ...
YouTube - Dipole Moment
Oct 5, 2009 ... The trigonal planar shape is symmetrical (bond dipoles cancel). ... Added to queue Electric Dipole Moments Theory Part 1by vkiledj12343 ...
Molecular Structure and Polarity
Molecules that possess a dipole moment are called Polar molecules (remember the ... substituted molecules; Asymmetric substitution giverise to a net dipole. ...
DIPOLE MOMENT ANALYSIS: [Print threshold: Net dipole >0.020 Debye ...
DIPOLE MOMENT ANALYSIS: [Print threshold: Net dipole >0.020 Debye]. NLMO bond dipole. NBO bond dipole. ------------------------- ------------------------ ...